The need for better profit margins in the refineries led to the search for more profitable crude oils, however in most cases these crudes could exhibit high contamination by different metals. A further strategy to improve the refinery profitability is the rise in the processing of heavier feedstocks (HVGO, KGO, DMO) and the increase in the catalytic cracking units’ atmospheric residue (ATR) percentage.
The fluid catalytic cracking unit feedstocks can contain a few contaminating metals, such as Vanadium, Nickel, Calcium, Iron among others. The increase in the processing of heavier crudes and residual feedstocks in the FCC unit rises the content of these undesired contaminating metals.
To determine the best operating strategy while securing the maximization of the unit profitability it is essential to know the effects of the feedstock nickel on the FCCU catalyst inventory.
During the catalytic cracking operation, the contaminating nickel present in complex organic molecules and paraffins of the feedstock is deposited on the catalyst, continuously poisoning the inventory, in opposition to the hydrocarbon molecules that are broken down into high-value added products resulting from contacting the catalyst.
Contrary to the action of Vanadium, that causes dealumination of the Y zeolite structure, destroying its crystallinity and collapsing its structure, nickel has no action on the zeolite activity. Nickel just tends to reduce to the metallic state in the riser, altering the reactions selectivity.
Nickel acts as a dehydrogenation agent increasing undesired by-products yields, hydrogen and coke. Besides nickel activity, the remaining metals, in a less pronounced way, also cause dehydrogenation, that is why the equivalent nickel follow-up is fundamental.

The best way to control the nickel effect is through the H2/CH4 dry gas ratio. Values up to 0.30 %v/%v of H2/CH4 point to low dehydrogenation activity, on its turn values above 0.50 %v/%v can indicate contamination of the inventory. A further important point to be considered is that the performance of the feed injecting nozzles can also affect the H2/CH4 dry gas ratio.
As can be observed in Table 1, the presence of nickel causes considerable conversion losses in the catalytic cracking unit. Conversion reduction is attributed mainly to the altered coke balance, where coke yield rises by metals and reduction in catalytic coke:
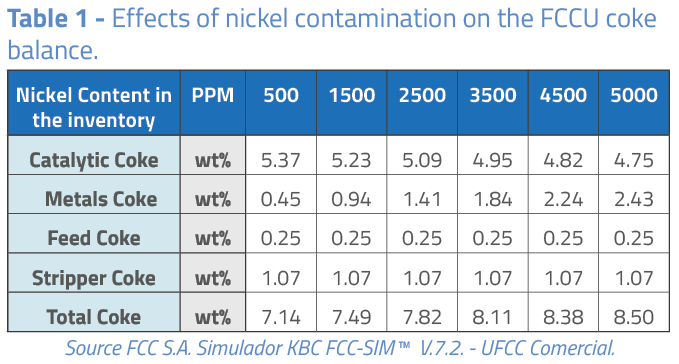
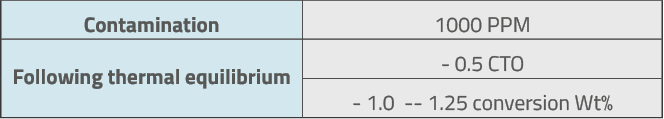
In general, and for a quick calculation, the following effects of the nickel activity on the catalytic cracking unit can be considered:

Following thermal equilibrium, CTO is reduced due to the increase in coke, reducing the unit conversion.
Besides the conversion loss, the increase in the dry gas H2 content can harm the wet gas compressor (WGC), since H2 exceedingly increases the dry gas volume, thus raising the volumetric flow and therefore, the speed at the WGC inlet. In case the speed increases a lot, the equipment may vibrate, being able to activate the protection system and leading to the catalytic cracking unit emergency stop.
The plots below show the effect of nickel on the conversion and profitability results of the catalytic cracking unit.
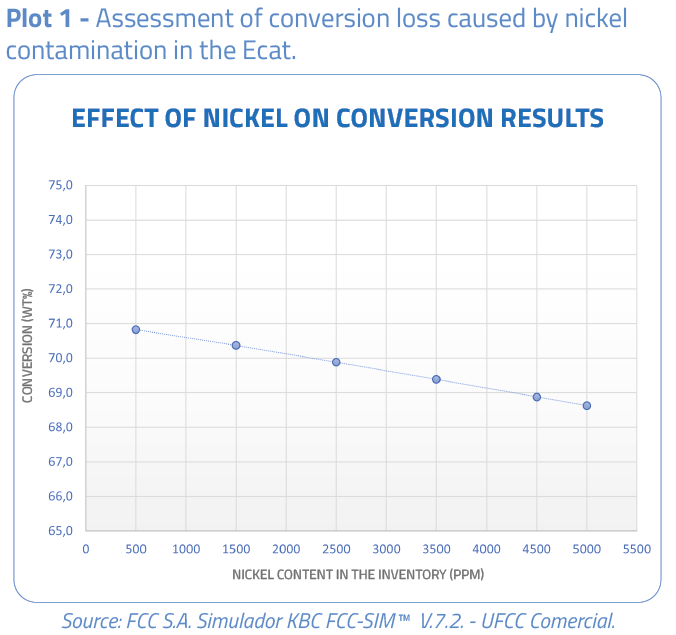
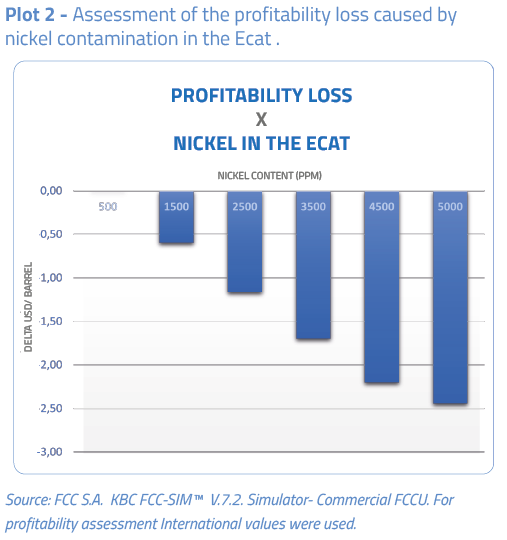
Actions to minimize the nickel effects on the catalyst inventory
- Utilization of trap additives: Antimonium-based additives can be used. However, in general these additives are highly toxic, requiring specific attention when using them, some countries having even banned their use. Besides their toxicity, antimonium can also increase NOx emissions, in a few cases, when combined with the use of combustion promoters it can rise the NOx emissions to values up to 4 times higher.
- Increased make-up: It is crucial to adjust composition in accordance with the flow and feedstock nickel content. The make-up adjustment is essential to minimize the impact of the unit profitability loss.
- Catalysts Technology: The catalyst formulation can contain special aluminas designed for capturing nickel, as are the technologies employed by Fábrica Carioca de Catalisadores S.A. The special aluminas developed by Fábrica Carioca de Catalisadores S.A. minimize the effects of nickel dehydrogenation, acting as a trap for the Ni2+ ions present in the feedstock as porphyrin-type complexes. These compounds, prevented from entering the zeolite Y pores, are decomposed on the alumina surface. The high temperatures to which the catalyst is submitted during the different FCC process steps promote the reaction of Ni2+ with alumina, leading to the formation of the NixAl2O3+x solid solution.
Conclusion:
In order to avoid losses in the refinery economic results while securing the maximum FCCU profitability it is essential to: effect the control and follow-up of the nickel contamination in the Ecat and employ mitigating measures to reduce the contamination effects.
FCC S.A makes available a portfolio of technologies suitable for the processing of feedstocks with high levels of metallic contaminants and provides its customers with the optimization service in the KBC FCC-SIM™ V.7.2 thermodynamic simulator, which is part of our core services. In case of interest you just have to contact one of our Technical Service engineers.


































What did you make of the publication?